This new section is devoted to publish synthetical procedures for obtaining fine chemicals from current commercial chemicals. The procedures, developed in our laboratories were perfected and simplified in order to produce them in a pilot plant scale.
April 2022; C.H. Gaozza & S. Olmo – Desynth S.A. Argentina
Diethyl Succinate
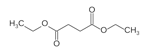
Place 57.0 g of commercial succinic anhydride, 160 ml of ethanol, 80ml of toluene and 0.7 ml of sulphuric acid in a 500 ml round-bottom flask with magnetic stirring and an 18 cm Hempel type-fractionating column distilling adapter and a condenser. The mixture is slowly heated with an oil bath until gets to 115°C. After anhydride is dissolved a azeotropic mixture of alcohol, toluene and water distils at 75°C. Continue the distillation until the thermometer rises to 78-79°C and then remove the column and replace it by a claisen adapter. Distil the remnant alcohol and toluene under vacuum from a water pump, after that distil the residual oil at 10 mm, boiling point 120-125°C
It yield 75 g (77%) of diethyl succinate.
Methyl methanesulfonate CH₃SO₃CH₃
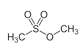
Place 40 ml of methanol in a 100 ml round bottom flask . Place it in a water bath with magnetic stirring at 20°C. With a dropping funnel add slowly (about 30 min) 17 ml of methanesulfonyl chloride then place a reflux condenser and heat the water bath until a mild boiling of the mixture for 30 min. Now remove the condenser and using a claisen distillation head the exceeding methanol is first distilled at normal pressure and the remainder under vacuum from a water pump. The water bath is replaced by an oil bath and the product distilled at about 10 mm; boiling point 135-138°C.
It yields 20g (83%) of methyl methanesulfonate.
Methanesulfonamide CH₃SO₂NH₂
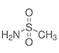
p-Toluesulfonyl chloride react with aqueous ammonia yielding p-toluensulfonamide almost quantitalively. But in the other hand, methanesulfonyl chloride only produces ammonium chloride and ammonium methanesulfonate. Methanesulfonamide only can be prepared with anhydrous ammonia in an organic solvent.
In a fume hood place a 120 ml round bottomed flask with magnetic stirring and water bath. Place in 10.0 g of methanesulfonyl chloride and 55 ml of toluene and heat the solution at 40-45º. Then from an ammonia gas cylinder equipped with a fine control valve send to the solution a mild stream of ammonia by means of a glass tube(4 to 5 mm inner diameter) holding above the stirring vortex during 2 hours (Warning!: this tube could be obtured easily by the reaction products). Cool to room temperature and filter off the white solid, a mixture of methanesulfonamide and ammonium chloride. Return it to the flask, add 8 ml of anhydrous ethanol. Heat and reflux the suspension for 15 minutes, cool to room temperature and filter off, wash the flask with 5 ml of anhydrous ethanol and add it to the filtrate. Using a vacuum rotaevaporator evaporate 12 ml of the solvent.
It yields 5.1 grams of Methanesulfonamide crystals. m.p. 84-86ºC. The product is suitable for most purposes.
4-Acetoxy-1-butanol
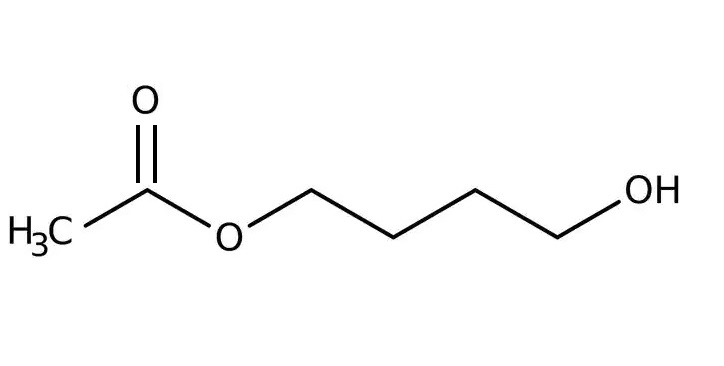
Monoacylation of difunctional compounds by acylating reactives always result in poor yields. We found a selective monoacetylation of 1,4-butanediol by means of a trans esterification reaction with ethyl acetate.
In a 50 ml conical flask place 0.16 g of sodium and 20 ml of 1,4-butanediol. Warm the mixture gently with continuous swirling until a clear solution is obtained. Place it in a 200 ml round bottomed flask with magnetic stirring and add another 25 ml of butanediol. Stir the mixture for 20 minutes and introduce 50 ml of ethyl acetate. Stir the turbid mixture until a clear solution is obtained. Allow the flask to stand overnight at room temperature. The reaction is completed by gently reflux heating for 2 hs. After cooling arrange the flask for distillation and collect the liquid (30 ml of ethanol with a remainder of ethyl acetate) with boiling below 78º and then arrange the flask for vacuum distillation of the remainder product (crude acetoxybutanol). It distils at 84-86º at 2-3mm, as a colorless oil. Yield 47 grams (70%).

